Photoplethysmography PPG, pronounced “FOTO-PLETH-ISS-MOG-RAFFY”, the Greek origins of the name mean to measure a volume change using light.
PPG is a method of measurement used in Biomedical Engineering to sense the blood pulsations under the skin and in organs using a light source and light detector. It is used in applications such as heart rate measurements and pulse oximetry.
Fundamental to the idea of photoplethysmography is the fact that light is observed to pass through tissue, this can be demonstrated simply by holding a light source such as a torch against a hand and being able to discern a faint red “glow” around the torch and on the other side of the hand (Rolfe, 1979).
Light traverses the tissues of the hand and photons are absorbed, scattered and reflected, the faint red glow we see is a result of these processes having varyingly different amounts of effects on different wavelengths of light until only the red hues are permissible. Utilising photo-detectors, an analysis of this light shows that the light being received varies with time and in synchronicity with the beating of the heart (Allen, 2007).
The observations made with a torch reveal that detecting photons from the light source can be done with two different methods of source-detector orientation. Where light is seen to pass all the way through the hand the photo-detector can be placed opposite the source, this is called transmission-mode photoplethysmography. This is the common technique used in ambulatory or bedside monitoring where a pulse oximeter (a device used to measure blood oxygen saturation utilising the principles of photoplethysmography) uses a finger probe attached to the patient.



The second method involves orientating the source and detector on the same plane, the glowing around the torch would then be detected as light that is back-scattered towards the sensor, this is called reflectance-mode photoplethysmography. Monitoring sites used are typically the forehead or the temple, however monitoring from any site is possible (Rolfe, 1979).
Reflectance mode has found a use in the research of new ways of monitoring PPGs from various anatomical sites (Kyriacou, 2006, Phillips et al., 2009, Phillips et al., 2010, Hickey et al., 2011, May et al., 2017, May et al., 2019), where the usage of a transmission-mode sensor is impractical.
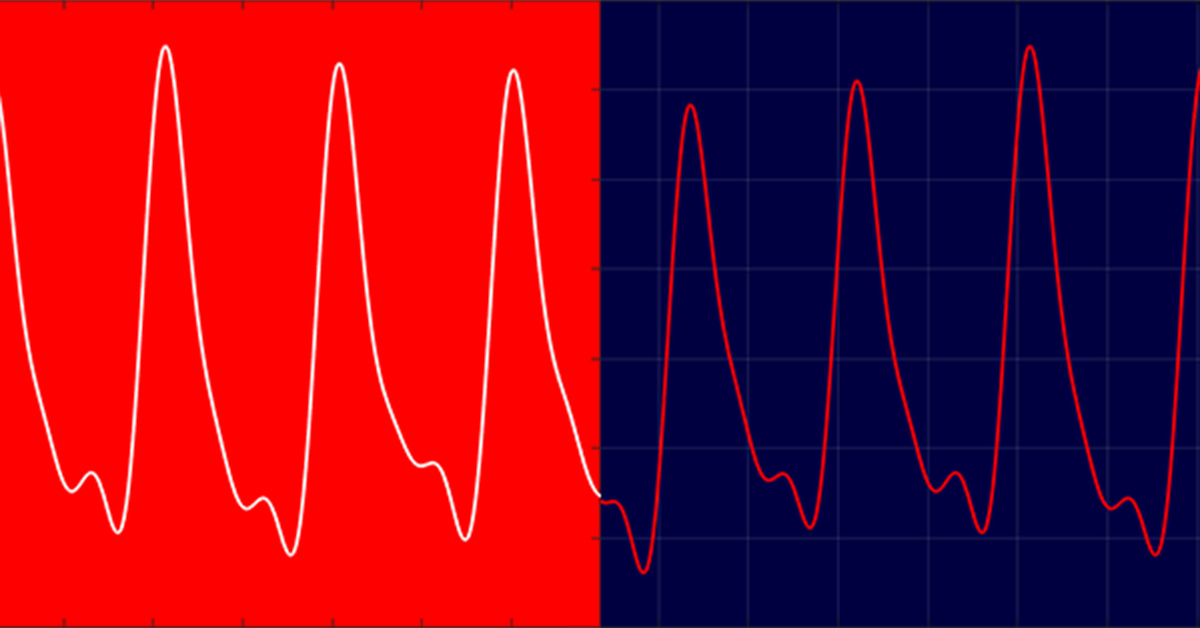
The properties of the PPG waveform, whether from transmission or reflectance mode, bare the same physical characteristics. As the heart contracts (systole, SIS-TOLI) blood is forced through the arterial system and a pressure wave is produced, which gives rise to an increase in blood volume at sites where these “pulses” can be physically observed.
During PPG monitoring the increase in blood volume can be attributed (although it is debated that this is not the only contributory factor to the PPG waveform) by a reduction in the light intensity that is being received by the photo-detector. This in-turn represents itself as a decrease in current output from the photodetector, and therefore a decrease in electrical voltage, when observed using an oscilloscope (assuming no signal inversion).
At diastole (DI-ASS-TOLI), the heart relaxes and the pressure wave diminishes and therefore so does the blood volume at the pulse sites. This manifests as a rise in electrical voltage on the oscilloscope as the amount of light penetrating through to the detector intensifies.
The PPG waveform is more commonly plotted with absorption rather than voltage; these are inversely proportional, so diastole is now represented by the trough of the waveform, and systole with the peaks. The actual morphology of the signal produced has a large DC offset with a vaguely sinusoidal riding waveform. The riding waveform is indicative of the process just described and has a distinct shape, with notches and a generally steeper increase at the systolic portions of the signal, the photoplethysmographic signal can be divided into two separate components;



DC PPG – Manifests as a perceptibly consistent voltage offset. Since light is always passing through tissue, some of this light is always absorbed by the skin, bone, other various tissues and venous blood. Signal extraction techniques can extract this component for further analysis, usually with simple low-pass filtering with a typical bandwidth of 0 – 0.5 Hz (Webster, 1997).
AC PPG – The pulsatile component of the signal and its origin comes from the beating of the heart as has been previously described. This rides on top of the DC PPG and only accounts for 1 – 2% of the entire signal. Like the DC part this can also be extracted for individual analysis using band-pass filtering with a typical bandwidth of 0.5 – 20 Hz (Webster, 1997).
For a historical perspective on Photoplethysmography you can read this article for further information.
References
- ROLFE, P. 1979. Non-invasive physiological measurements, Academic Press
- ALLEN, J. 2007. Photoplethysmography and its application in clinical physiological measurement. Physiological Measurement, 28, R1-39.
- KYRIACOU, P. A. 2006. Pulse oximetry in the oesophagus. Physiological Measurement, 27, R1-35
- PHILLIPS, J. P., et al. 2009. Investigation of photoplethysmographic changes using a static compression model of spinal cord injury. Conference Proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference, 2009, 1493-1496
- PHILLIPS, J. P., et al. 2010. Cerebral arterial oxygen saturation measurements using a fiber-optic pulse oximeter. Neurocritical Care, 13, 278-285
- HICKEY, M., et al. 2011. Investigation of photoplethysmographic signals and blood oxygen saturation values obtained from human splanchnic organs using a fiber optic sensor. Journal of Clinical Monitoring and Computing.
- MAY, J. M., et al 2017. A novel fontanelle probe for sensing oxygen saturation in the neonate. Biomed. Phys. Eng. Express 3 015023
- MAY, J. M., 2019. A Novel Photoplethysmography Sensor for Vital Signs Monitoring from the Human Trachea. Biosensors 2019, 9, 119
- WEBSTER, J. G. 1997. Design of pulse oximeters, Institute of Physics Pub.

Pingback: What are those Lights on my Smart Watch? What do they do?